Making drug delivery available for every person on earth
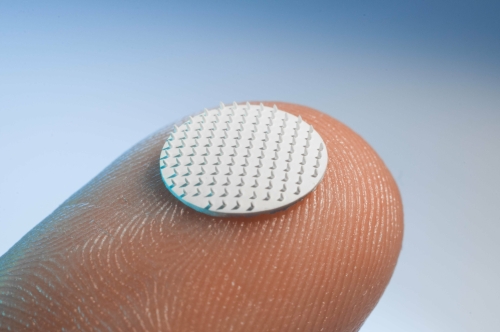
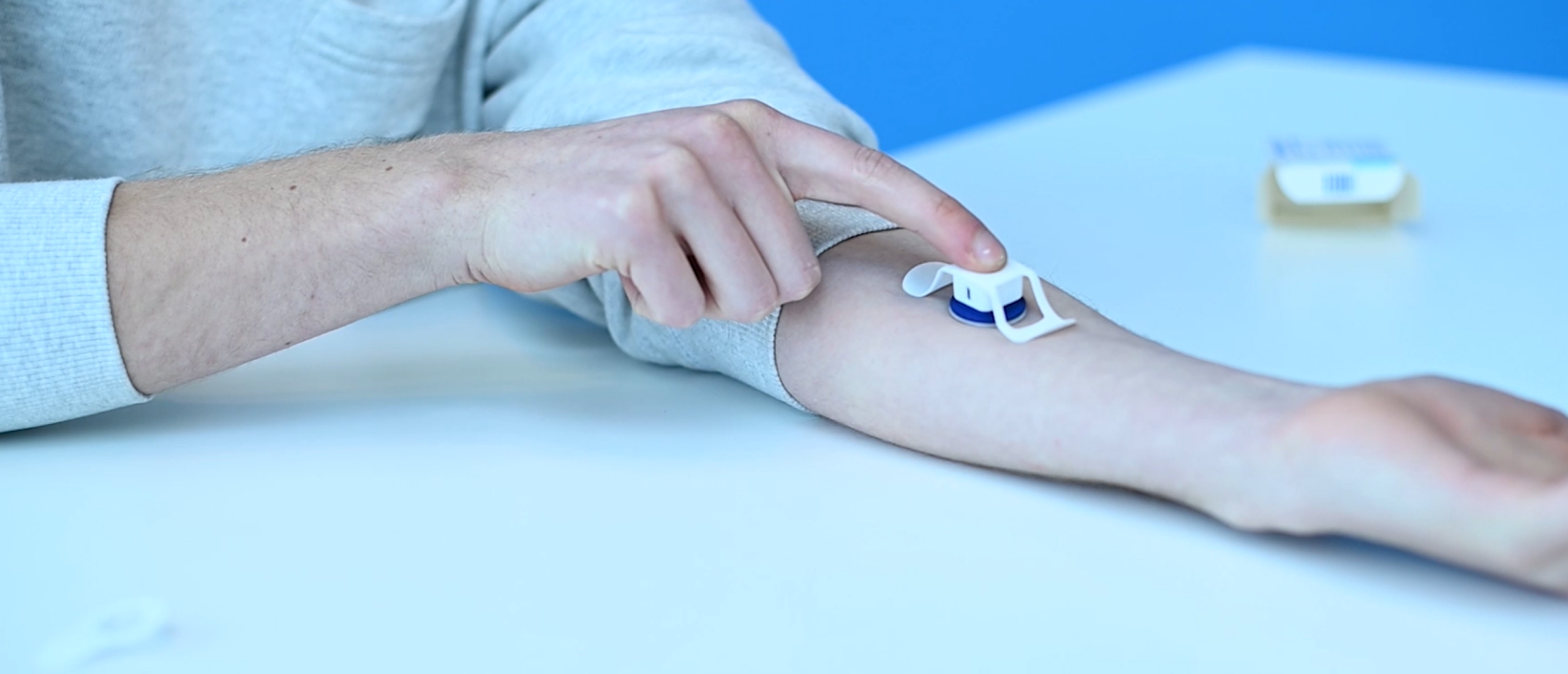
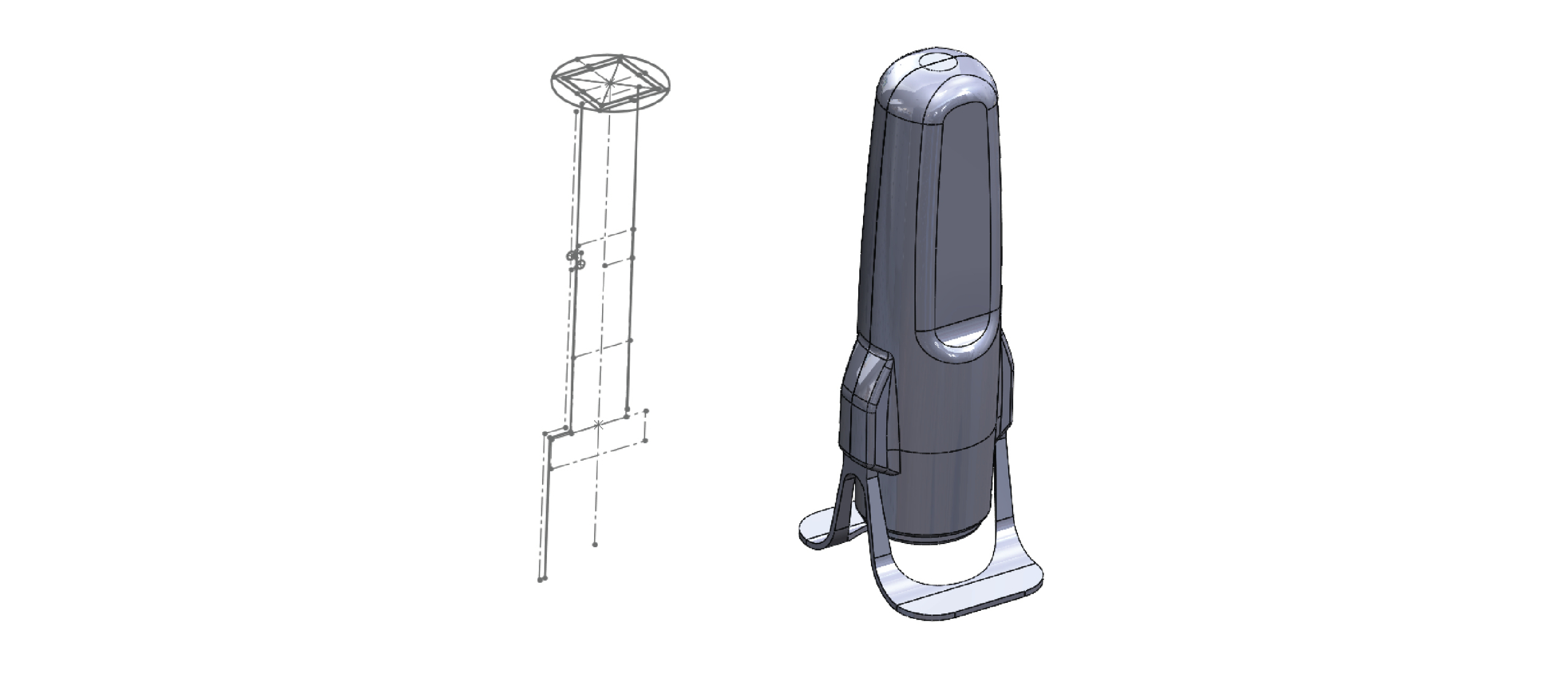
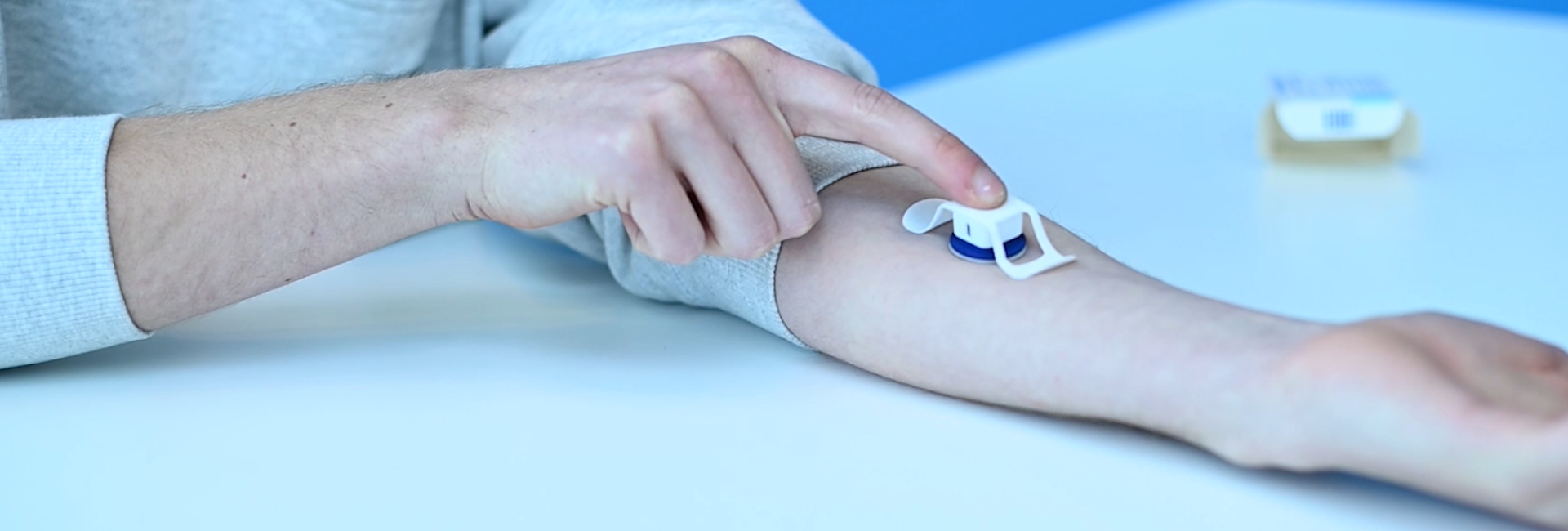
We do this by unlocking the potential of microneedle technology as a superior new drug delivery system compared to hypodermic needles. This novel technology uses a collection of very small needles of less than 1mm in length, containing the drug or vaccine, mounted on a backplate. With our applicators, users can administer the vaccines and drugs themselves painlessly, effectively and consistently. The microneedles are not required to be stored or distributed under refrigerated conditions, thereby eliminating the logistical cold chain.